Headline
Simultaneous vaccination of elderly using Sinovac vax set
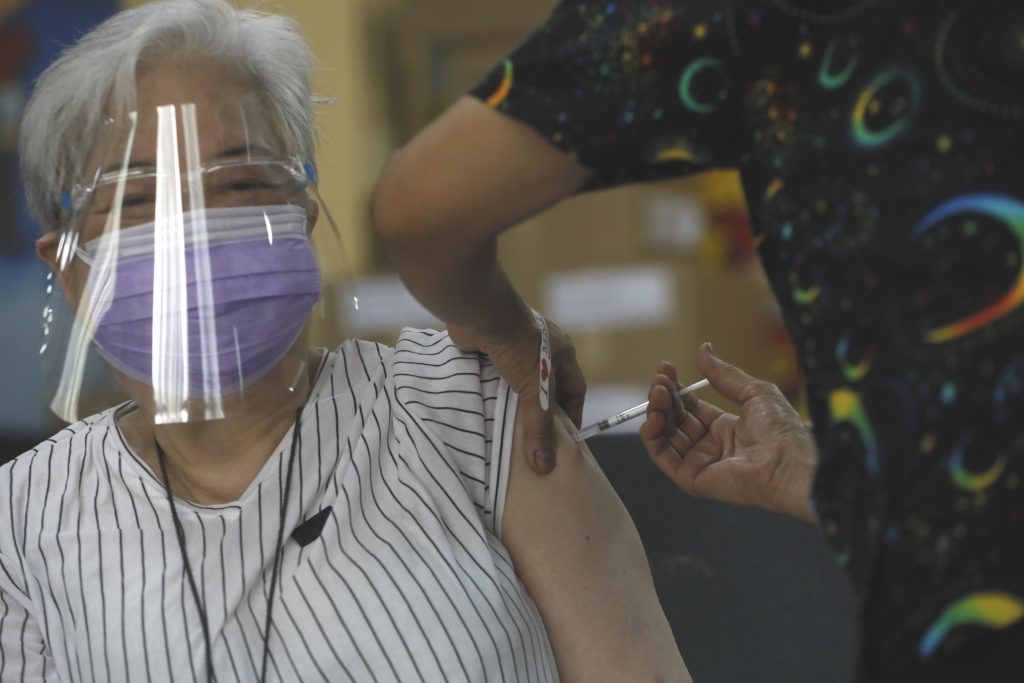
MANILA – A simultaneous implementation of voluntary vaccination of senior citizens using Sinovac’s CoronaVac Covid-19 vaccines is set to begin on April 12.
This, after the Food and Drug Administration (FDA) approved the use of CoronaVac on senior citizens based on reports from other countries shared by the vaccine expert panel.
Undersecretary Myrna Cabotaje, National Covid-19 Vaccination Operations Center chairperson, said the vaccination will begin next week to give all regional and local vaccination operation centers enough time for the logistical preparations of the vaccination sites and organize the scheduling of recipients.
“In preparation for simultaneous implementation next week, we are advising all implementers to update its scheduling to include senior citizens for voluntary CoronaVac vaccination and wait for the issued guidelines for proper guide on its use,” Cabotaje said in an advisory she signed on Wednesday.
The Department of Health (DOH) will be issuing implementing guidelines on the use of CoronaVac to the elderly, consistent with the advice of the FDA.
“After considering the recommendation of the experts and the current situation of high Covid-19 transmission and limited available vaccines, the FDA is allowing the use of Sinovac on senior citizens,” FDA Director-General Eric Domingo said in a separate statement.
Domingo emphasized all factors for the approval were considered.
“Vaccination should be preceded by an evaluation of the person’s health status and exposure risk to assure that benefits of vaccination outweigh risks,” Domingo noted.
The FDA initially recommended the use of Sinovac’s CoronaVac on clinically healthy individuals aged 18-59 years old.
It earlier approved the Emergency Use Authorization (EAU) for Sinovac Biotech.
In a joint statement, the FDA and DOH said the health status of the elderly must be evaluated first, before administering them with CoronaVac jabs.
“The DOH and FDA emphasized that the vaccination of senior citizens using CoronaVac should strictly be preceded by careful evaluation of the person’s health status and exposure risk,” the statement reads. “There is a Phase I/II safety and immunogenicity data for seniors but efficacy data is not yet sufficient to establish vaccine efficacy.”
Vaccine czar Sec. Carlito Galvez Jr. earlier said about 9 million senior citizens are listed in the government’s priority master list on the Covid-19 vaccination program.
Some local government units have started inoculating senior citizens using the AstraZeneca vaccine on Mach 31.
On Thursday, the city government of Manila started administering the Sinovac vaccines to its senior citizens.
The country has so far received a total of 2 million doses of CoronaVac, including 1 million vials donated by the Chinese government.
On March 29, President Rodrigo Duterte welcomed the arrival of the initial 1 million doses of the 25 million CoronaVac vaccines purchased by the government from Sinovac Biotech.
The Philippines is expecting to receive an additional shipment of 1.5 million doses of Sinovac’s CoronaVac vaccines this month, 2 million doses by May, another 4.5 million doses in June, and 3 million doses in July.





















