Health
A million deaths from coronavirus: seven experts consider key questions

Cumulative deaths from COVID-19 per million of population (dpm), are very unevenly distributed across Europe (see map below) ranging from 7dpm in Slovakia to 856dpm in Belgium. A wedge of relatively lightly affected countries extends from Finland southwards to the northern Balkans. (Pixabay photo)
The pandemic has reached a grim milestone: one million people have now died of COVID-19, according to Worldometers.
On January 13, we published “Mystery China pneumonia outbreak likely caused by new human coronavirus” by Connor Bamford, a virologist at Queen’s University Belfast. Since then, we have published more than 3,500 articles on the now not-so-novel coronavirus, officially named Sars-CoV-2. Despite this huge output from the world’s leading experts, we have merely skimmed the surface of all there is to know about this perplexing pathogen. So much remains a mystery.
At this important juncture, we asked several experts from different fields what their burning question about the coronavirus is. Here is what they said:
Connor Bamford, Research Fellow, Virology, Queen’s University Belfast
How did Sars-CoV-2 enter the human population?
We must understand how Sars-CoV-2-like viruses jump into humans if we are to stop the next pandemic, as we do for influenza. Although originally thought to have emerged in the Huanan Seafood Wholesale Market in December 2019, the earliest patient had no link to the market suggesting the virus had emerged before then. How did this happen?
Read more:
Mystery China pneumonia outbreak likely caused by new human coronavirus
Since the original investigations into the beginnings of Sars coronaviruses in 2002, horseshoe bats in south-east Asia have been implicated as the reservoir hosts, and a virus (RmYN02) that is extremely similar to Sars-CoV-2 has already been found in bats. However, similar viruses have also been found in pangolins, raising the possibility that Sars-CoV-2 may not have jumped directly from a bat.
Also, Sars-CoV-2 has already spread to cats, dogs, tigers and mink, and for Sars-CoV-1 (the virus that caused the 2002-04 Sars epidemic), farmed civet cats and raccoon dogs acted as intermediate hosts, bringing a bat virus into proximity to humans. It is possible that Sars-CoV-2 is a generalist virus, capable of spreading through a wide range of species.
With the increase in contact between humans and wildlife, zoonoses are becoming an ever-growing threat. We must be vigilant. An important step now is to figure out the events that led Sars-CoV-2 to go from bat to human.
Sarah Caddy, Clinical Research Fellow, Viral Immunology, University of Cambridge
How can we tell if someone is protected from Sars-CoV-2?
The immune response to Sars-CoV-2 infection aims to eliminate the virus from the body. Many studies have carefully described the various stages of the immune response after initial infection, but we do not know which aspects of immunity are essential for preventing repeat infections. What are the relative roles of different types of antibodies, or the importance of different T cell subsets?
An important goal of Sars-CoV-2 immunological research is, therefore, to identify which immune component (or components) can show a person is protected from future infection. Such a marker would be termed a “correlate of protection”.
The ability to measure an accurate correlate of protection would be valuable for two reasons. First, it could tell us whether someone who has recovered from COVID-19 is likely to get re-infected. Second, identifying an easily measurable correlate of protection would be helpful for vaccine trials – it could speed up the evaluation of vaccine efficacy.
However, identifying good correlates of protection for other coronaviruses has proven notoriously difficult. Useful results have previously only been generated when volunteers were experimentally infected with viruses. The first human Sars-Cov-2 challenge studies are now due to begin early next year, so it is hoped that this will enable correlates of protection to be found more rapidly.
Read more:
Coronavirus: why I support the world’s first COVID vaccine challenge trial
Derek Gatherer, Lecturer and Fellow of the Institute for Social Futures, Lancaster University
How can we explain the extreme geographical variation in COVID-19 mortality rates?
Cumulative deaths from COVID-19 per million of population (dpm), are very unevenly distributed across Europe (see map below) ranging from 7dpm in Slovakia to 856dpm in Belgium. A wedge of relatively lightly affected countries extends from Finland southwards to the northern Balkans.
There are similar pockets of low COVID-19 mortality on other continents, notably south-east Asian countries. Could the populations of low mortality countries have some cross-immunity to Sars-CoV-2 generated by recent exposure to another coronavirus – the obvious candidates being the milder “common cold” coronaviruses: 229E, NL63, OC43 or HKU1?
A hint that this may be the case is provided by the observation that antibodies from the original 2003 Sars patients have some binding to coronaviruses 229E, NL63 and particularly OC43. But so little attention has been paid to seasonal coronaviruses, indeed, to seasonal non-flu respiratory infections, in general, that relevant clinical field data is extremely sparse and often old (for instance, one-third of residents of Hamburg had antibodies to coronavirus OC43 in 1975 or 58% of Hungarians sampled five years later).
We urgently need more lab studies to understand how much cross-immunity coronaviruses confer on each other, while population studies are needed to determine the prevalence of coronavirus antibodies, not just to Sars-CoV-2 but also its milder yet potentially significant cousins.
Serology – the study of antibody prevalence – has long been the Cinderella of virology compared with the more glamorous world of genome sequencing, but its significance and the consequences of its neglect are now becoming apparent.
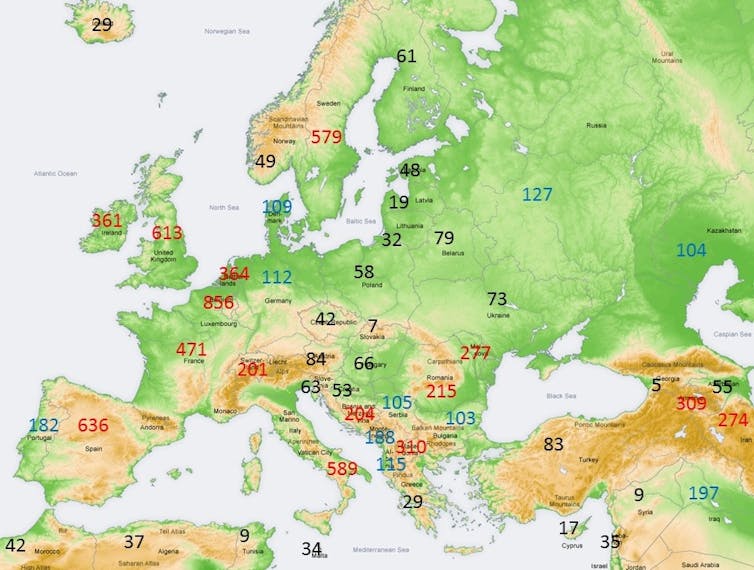
By San Jose – own map, based on the Generic Mapping Tools and ETOPO2 (annotated by DG). Data from WHO Epidemiological Update., CC BY-SA
Anne Moore, Senior Lecturer in Biochemistry and Cell Biology, University College Cork
For a vaccine, what does success look like in the short versus long term?
The endgame to the COVID-19 pandemic requires the identification and manufacture of a safe and effective vaccine and a subsequent global immunisation campaign.
Candidate Sars-CoV-2 vaccines were rapidly developed based on years of vaccine development efforts. The unprecedented and significant input of global funding into this pandemic vaccine effort can only buy so much time for trials to succeed or fail. A successful trial needs the virus to be circulating in the community so we can determine how many vaccinated people (versus those receiving a placebo) become infected.
Short-term success will show that a safe vaccine will provide at least 50% protection. And if we see short-term success, what does long-term success look like?
The biggest question is, what is the duration of protection? If it is short-lived, then how do we boost immunity back to protective levels? How do we figure this out without relying on a traditional empirical approach? If there isn’t short-term success, then how do we ensure that global commitment is maintained to prevent Sars-CoV-2 vaccines from ending up in the same situation as terminated vaccine efforts for Sars? There will be another pandemic; we need a long-term vision and commitment to have short-term future success.
Susan Michie and Robert West, Professors of Health Psychology, UCL
How can COVID-safe behaviour become embedded in people’s lives?
It looks as though COVID-19 will be with us for the foreseeable future. We will all have to adopt a range of behaviours to keep ourselves from getting infected or infecting others. We know what these are: the question is how they can become embedded in our lives?
The behaviours include keeping a greater physical distance from others; carrying a COVID kit (face mask, hand sanitiser and tissues) whenever we are outside the home; wearing a face mask properly in indoor public areas and storing or disposing of it safely; disinfecting hands and surfaces after possible contamination; catching coughs and sneezes in tissues; never touching our eyes, nose or mouth unless we know our hands are clean; avoiding or leaving unsafe situations, such as poorly ventilated indoor areas where there are lots of people; getting vaccinated; and staying at home and getting tested if we have symptoms.
The challenge is how to get these adopted at scale and maintained over time, in other words, embedded in people’s lives as routines and habits. This requires an understanding of what maintains and changes human behaviour. We need to equip people with the skills to develop routines that can become habits over time, provide the time and social and environmental support to achieve this and motivate them to use these opportunities.
David Hunter, Richard Doll Professor of Epidemiology and Medicine, University of Oxford
What is the full spectrum of health consequences of COVID-19 infection?
We now have good data on deaths from COVID-19 infection, showing an astonishing increase in risk of death with increasing age. This contrasts with the 2009 H1N1 flu epidemic, in which the aged were relatively less affected, and reminds us that we have a great deal more to learn about this virus.
While most of the focus has been on deaths, small studies of COVID-19 survivors discharged from hospital suggest that many do not return to their baseline health status. We know little about “long COVID” among those who did not require hospital admission, despite many individual reports of recurrent bouts of fever, fatigue, and a wide range of other symptoms.
Follow-up of COVID-19 patients suggest evidence of damage to the heart, lungs and other organs that may cause problems in the future, and there is some evidence that this may be true even among those with mild symptoms. Many viral infections can cause undiagnosed pathology, but severe long-term effects are relatively uncommon. If these effects are more common for COVID-19, however, then an exclusive focus on deaths means that we will not be considering the full costs of failing to control the epidemic, nor the full benefits of doing so.
Studies have started among patients after discharge from hospital. We urgently need well-controlled studies among the majority of those infected who did not need hospitalisation in case we are only seeing the tip of the COVID iceberg.![]()
Sarah L Caddy, Clinical Research Fellow in Viral Immunology and Veterinary Surgeon, University of Cambridge; Anne Moore, Senior Lecturer in Biochemistry and Cell Biology, University College Cork; Connor Bamford, Research Fellow, Virology, Queen’s University Belfast; David Hunter, Richard Doll Professor of Epidemiology and Medicine, University of Oxford; Derek Gatherer, Lecturer, Lancaster University; Robert West, Professor of Health Psychology and Director of Tobacco Studies, UCL, and Susan Michie, Professor of Health Psychology and Director of the UCL Centre for Behaviour Change, UCL
This article is republished from The Conversation under a Creative Commons license. Read the original article.





















