Health
How tiny gold particles injected into tumours could improve radiation treatment for cancer
Cancer is the second leading cause of death globally. In 2018, there were 18.1 million new cases and 9.5 million cancer-related deaths worldwide. By 2040, the number of new cancer cases per year is expected to rise to 29.5 million and the number of cancer-related deaths to 16.4 million.
Approximately 50 per cent of all cancer patients can benefit from radiotherapy in the management of their disease. About half of those patients are diagnosed early enough that their cancer may be curable. For many cancers including breast, prostate, cervix, head and neck, lung and brain cancers, curative treatment includes radiation therapy. However, because radiotherapy destroys healthy cells as well as tumour cells, doses are limited.
Radiotherapy, also called radiation therapy, is used alone to treat cancer or with other treatment options such as chemotherapy and surgery. It may also be used to shrink the tumour before surgery. In radiotherapy, tumour cells — which divide much faster than other surrounding healthy cells — are destroyed by damaging their DNA.
Side-effects limit radiation dose
The limiting factor in radiotherapy is that doses high enough to try to cure high-risk (locally advanced) non-metastatic tumours also damage surrounding normal tissues. Currently, we are at the limit of radiotherapy dose that can be given to patients. To further improve survival, there is a need for new methods that enhance radiation effectiveness while reducing side-effects.
One way to accomplish this is by making tumour cells more sensitive to radiation, so those cells are more easily damaged by radiation therapy. Using gold nanoparticles as radiosensitizers has shown promising results. These gold nanoparticles can be introduced intravenously to accumulate in the tumour by exploiting the faulty walls of the tumour’s blood vessels, which tend to be leaky because of fast growth.
Gold nanoparticles interact with X-ray photons used in radiation treatment which produces electrons, which then interact with water molecules to produce free radicals. These free-radicals can damage cells, lowering the survival of those cells.
Understanding the complex biological system present in and around the tumour is essential for optimizing the use of the radiosensitizing GNPs, as outlined by a consortium of labs, including our own nanoscience and technology development laboratory at University of Victoria.
Targeting interactions inside the tumour
In this work, we discuss the importance of looking into which cellular components within the tumour microenvironment take up the gold nanoparticles and become radiosensitized. We are particularly interested in cells called activated fibroblasts, which are associated with wound healing and have anti-tumourogenic properties, meaning they help fight tumour growth.
However, activated fibroblasts can be recruited by the tumour cells, and become cancer-associated fibroblasts (CAFs). Instead of anti-tumourigenic properties, CAFs promote the proliferation and metastasis of tumours.
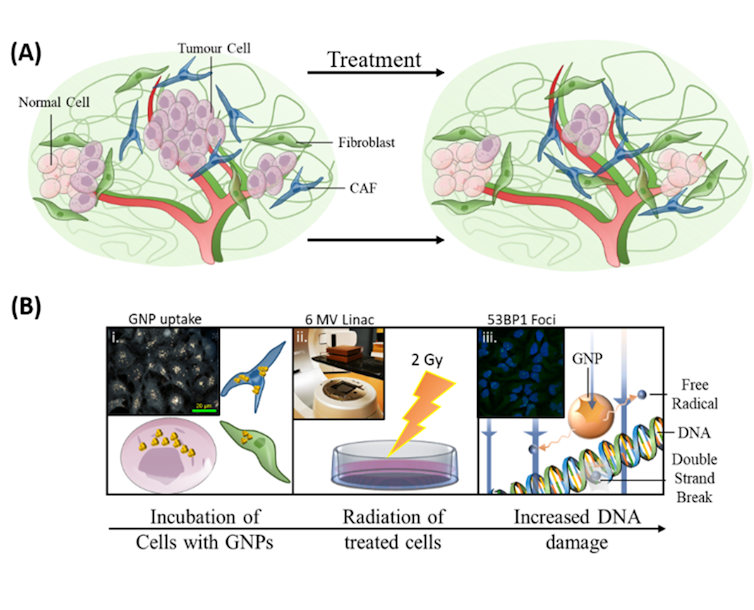
The function of CAFs supports the idea that tumours are “wounds that do not heal,” and targeting CAFs may prove beneficial towards improved cancer treatment outcomes.
As illustrated in the image above, our research on incorporating gold nanoparticles into current radiation treatment protocols had three goals: to enhance killing of tumour cells, to target CAFs and to protect fibroblasts.
For radiosensitizing to be effective in improving radiation treatment, the cells targeted by the treatment (the ones associated with cancer growth) need to have high uptake of the radiosensitizing particles, while the beneficial cells need to have a low uptake. This makes the targeted cells are more easily destroyed by radiation therapy at doses that patients can tolerate.
These results using 3D tumours grown in the lab are very encouraging. The CAFs had the largest uptake of the gold nanoparticles per cell, with almost triple that of cancer cells, while fibroblasts had a relatively small number. This also translated to a larger increase in DNA damage in the CAFs compared to the other cell types, reducing the activity of the CAFs and slowing tumour growth.
This difference in DNA damage due to selective targeting of cancer-associated cells over normal cells may allow gold nanoparticles to be an effective tool in future cancer radiation therapy, helping to minimize damage to normal tissue while improving local radiation therapy dose to the tumour.
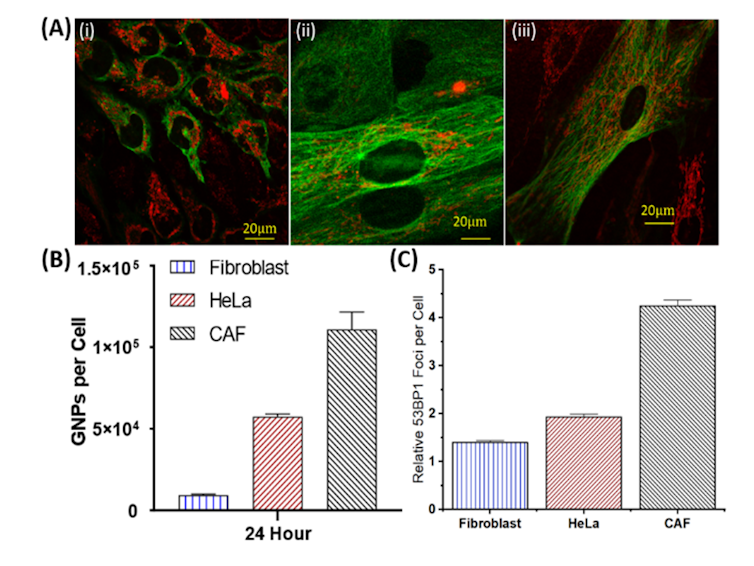
This study showcases that using gold nanoparticles as a radiosensitizer allows more damage to be propagated to the CAFs, an element that has shown to be largely influential to the progression of cancer. We believe that this work will be a building block towards a more effective treatment regime in the near future. Building a model that can accurately represent the different interactions taking place inside the tumour’s microenvironment is essential to improving treatment results for patients.
Devika Basnagge Chithrani, Associate professor, Physics and Astronomy/Medical physics, University of Victoria
This article is republished from The Conversation under a Creative Commons license. Read the original article.





















