Headline
2 dead due to Dengvaxia vaccine: PAO
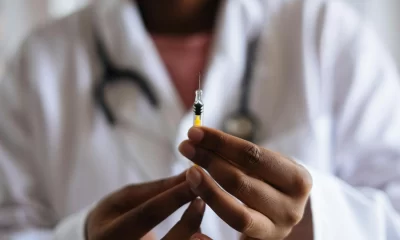
In their seven-page affidavit made by De Guzman, said their child received a shot of Dengvaxia on April 16, 2016 through a school vaccination project at Sisiman Elementary School (Photo by MIMS)
MANILA — The Public Attorneys Office (PAO) on Tuesday confirmed that there were two victims who died due to Dengvaxia, the world’s first-ever dengue vaccine which was found to cause health risk to individuals with no prior dengue infection.
In a press conference held in Manila, PAO chief Atty. Persida Rueda-Acosta and the Volunteers Against Crime and Corruption (VACC) presented the parents of Anjielica Pestilos and Christine Mae De Guzman, both 10, who died due to severe dengue months after receiving the world’s first dengue vaccine.
Acosta said that parents of Christine, Nelson and Marivic De Guzman, have already made their affidavit.
In their seven-page affidavit made by De Guzman, said their child received a shot of Dengvaxia on April 16, 2016 through a school vaccination project at Sisiman Elementary School.
“Habang kinukunan kami ng impormasyon, sinabihan kami pasalamat kayo binigyan tayo ng gobyerno ng libreng anti-dengue vaccine. Saprivate (hospital) po kasi may bayad na mahal aabot ng PHP4,500 to PHP5,000 (While we were being asked for information, we were told that we should be thankful to the government because the anti-dengue vaccine is free. If we will get it from private hospitals, it would cost around PHP4,500 to PHP5,000.),” read the affidavit.
The parents said the vaccine had no immediate effect to their child. However, after six months or on Oct.
11, 2016, their child complained of headache and had high fever. After two days, she suffered from severe stomach pain. She was then taken to the Mariveles Health Service Cooperative Hospital (MAHESECO) where she was diagnosed of having dengue.
As her condition worsened, De Guzman was transferred to the Intensive Care Unit of the Bataan Provincial Hospital where she had a blood transfusion. She also complained of difficulty breathing. She died on Oct. 15, 2016.
Apart from Christine, Acosta said Pestilos, died just last December 6 at the East Avenue Medical Center in Quezon City.
“Dalawang kaso itong inimbestigahan ng forensic laboratory (The forensic laboratory is investigating these two cases),” said PAO forensic laboratory chief, Dr. Erwin Erfe.
“In both cases, the victims never suffered previous dengue exposure, both were vaccinated with Dengvaxia, and have died from severe dengue,” he said in Filipino.
Though the diagnosis on the clinical abstract of Pestilos showed she suffered severe systemic lupus elythematosus, Erfe said they reviewed her clinical abstract and the pictures of her cadaver.
Like Christine, Dr. Erfe said Anjelica has not previous exposure to dengue.
“When we reviewed her clinical abstract, there is a manifestation that Anjelica died of severe hemorrhagic dengue,” Dr. Erfe said during the press conference.
He said the child has edema, rashes, bleeding and low platelet and WBC count.
“Merong manifestation na severe hemorrhagic dengue itong batang ito (She manifested severe hemorrhagic dengue),” he explained.
The VACC called the immunization program “worse than any heinous crime,” and urged the DOH to set up help desks to receive complaints from concerned parents and also vowed to help them seek compensation for the families whose children may have received potentially risky anti-dengue shots.
The VACC is also pushing for the conduct of investigation in order to hold those responsible for the mass vaccination.
The DOH said around 10 percent of the over 700,000 school children who received the shots were at risk to a “severe” case of the disease, prompting DOH Secretary Francisco Duque III to order the suspension of the dengue vaccination program pending recommendation on further action from experts from the WHO.
Pharmaceutical firm Sanofi Pasteur recently issued an advisory to the public that its product Dengvaxia is effective for people who have had dengue prior to immunization but creates a risk of a “severe” case of dengue for people who have not yet had dengue.
Prior to this, Philippine health officials are under fire for allegedly acting with undue haste by allowing the PHL to be the first Asian country to approve the vaccine for individuals aged 9 to 45 years old in December 2015.