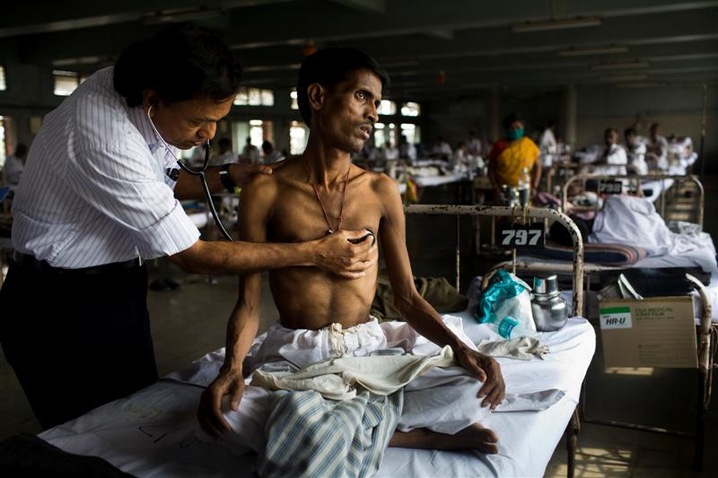
DAR ES SALAAM—Tanzanian scientists are conducting clinical trials on shorter courses of tuberculosis (TB) treatment which would now be more user-friendly and more effective to the patients, the scientists have said.
The scientists from the Ifakara Health Institute (IHI)—the east African-nation’s leading medical research facility –said the new treatment would help cut down the number of patients who drop out of treatment because of the aversive and long-duration medications that last up to six months.
Patients suffering from tuberculosis in Tanzania have been enduring the pains of being put on long-term medications against the disease until they recover but the new dosage could now last less than three months.
For many years, researchers on TB in Tanzania have warned that cases of patients who quit TB treatment are behind the increasing burden of drug-resistance in the country and the high transmission rates of the disease in communities.
Paul Smithson, IHI chief knowledge officer, said local researchers were now collaborating with international ones to try and come up with new shorter regimes of the drugs which would also contain more effective treatment substances against the disease.
“In the next four to five years, the mode of treatment for TB would enter a new era in Tanzania and the world at large,” Smithson told a news conference at IHI research center in the country’s historical town of Bagamoyo.
“Currently IHI is collaborating with the Global Alliance for TB Drug Development in a drug trial which will help come up with a drug combination known as bedaquilline, pretonamid and pyrazinamide,”he added.
Smithson said the new combination of the drugs, whose trial in Tanzania was now at phase II, was one of its kind, adding that in its new form, it will be able to tackle both drug-resistant tuberculosis and non-resistant tuberculosis.
This comes as the fourth clinical trial on TB drugs among a series of more others done at IHI. For the past 10 years, IHI has been also conducting surveillance studies on TB in rural and urban parts of the country, according to the IHI Director, Salim Abdullah.
Abdullah said that in the near future, the institute would also embark on testing another new TB combination of drugs in a phase III clinical trial.
Thomas Zoller, a team leader in TB research at IHI, said that if the other new combination of drugs known as moxifloxacin, pretonamid and pyrazinamide was successful, that would mean an important step in the efforts to produce less aversive but more effective medications for TB patients in Tanzania.
Studies show that one patient with TB can infect 10 to 15 others in one year. This, according to the experts, means that those patients who remain half-treated in the communities would lead to an increased TB burden in future.
Tanzania has been recording about 63, 000 TB patients each year for the consecutive past five years—making the country rank number six among African nations with the highest TB burden on the World Health Organization’s scales.