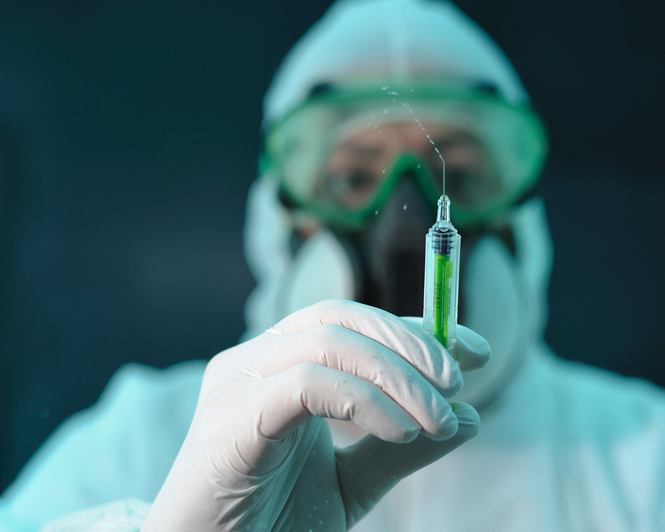
MADRID — Spain has imported a U.S.-made experimental Ebola drug to treat a Spanish missionary priest evacuated from Liberia last week after testing positive for the killer virus.
The Health Ministry announced Monday that the ZMapp drug, made by Mapp Biopharmaceutical Inc. of San Diego, was obtained in Geneva this weekend and brought to Madrid to treat Miguel Pajares. The 75-year-old priest was placed in isolation Thursday at Madrid’s Carlos III Hospital.
There is no known cure or licensed treatment for Ebola, which has killed more than 1,000 people in the current outbreak in West Africa. The World Health Organization has called the Ebola outbreak – which emerged in Guinea in March and has since spread to Liberia, Sierra Leone and possibly Nigeria – an international health emergency and urged nations worldwide to donate resources to battle the disease.
The ethical questions surrounding experimental Ebola drugs and vaccines were being debated Monday during a teleconference of medical ethicists and other experts organized by the U.N. health agency.
Two Americans diagnosed with Ebola in Liberia and evacuated back to the United States have been treated with the drug. One of them, Dr. Kent Brantly, said last week that his condition was improving and the husband of the aid worker being treated with Brantly said the same thing. Both are in isolation at an Atlanta hospital.
Spain said it obtained permission from the laboratory developing the drug and, under an agreement between WHO and the Doctors Without Borders charity group, imported the drug from Geneva where it said a dose had been available. The ministry said Spain sought the drug under legislation permitting use of unauthorized medication in patients suffering from a life-threatening illness who cannot be treated satisfactorily with any authorized drug.
Despite Spain’s statement, WHO spokesman Gregory Hartl told The Associated Press on Monday that the U.N. agency had no role in helping Spain obtain the experimental drug.
At least one country in West Africa has expressed interest in the experimental drug. Nigeria’s health minister, Onyenbuchi Chukwu, said last week he had asked U.S. health officials about access but was told the manufacturer would have to agree.
Dr. Tom Frieden, director of the U.S. Centers for Disease Control and Prevention, said “there are virtually no doses available,” a CDC spokesman said last week, before the announcement that Spain was also using the drug.
Because the ZMapp drug has never been tested in humans, scientists say there’s no way to tell if it has made any difference to the two American aid workers who have so far received it.
The drug is a mixture of three antibodies engineered to recognize Ebola and bind to infected cells so the immune system can kill them. Scientists culled antibodies from laboratory mice and ZMapp’s maker now grows the antibodies in tobacco plants and then purifies them. It takes several months to even produce a modest amount of the drug.
Nigerian health authorities, meanwhile, confirmed another Ebola case Monday, a nurse who was treating Patrick Sawyer, the Liberian-American who flew into the country with the disease and died of it last month. That brings the locally confirmed Ebola cases in Nigeria to 10, including two who have died, Sawyer and another nurse. Nigerian authorities have 177 contacts of Sawyer now under surveillance.
WHO has not yet confirmed the Ebola cases in Nigeria.
Medical writer Cheng reported from London.