News
Admin complaint vs. 13 DOH execs over Dengvaxia mess filed
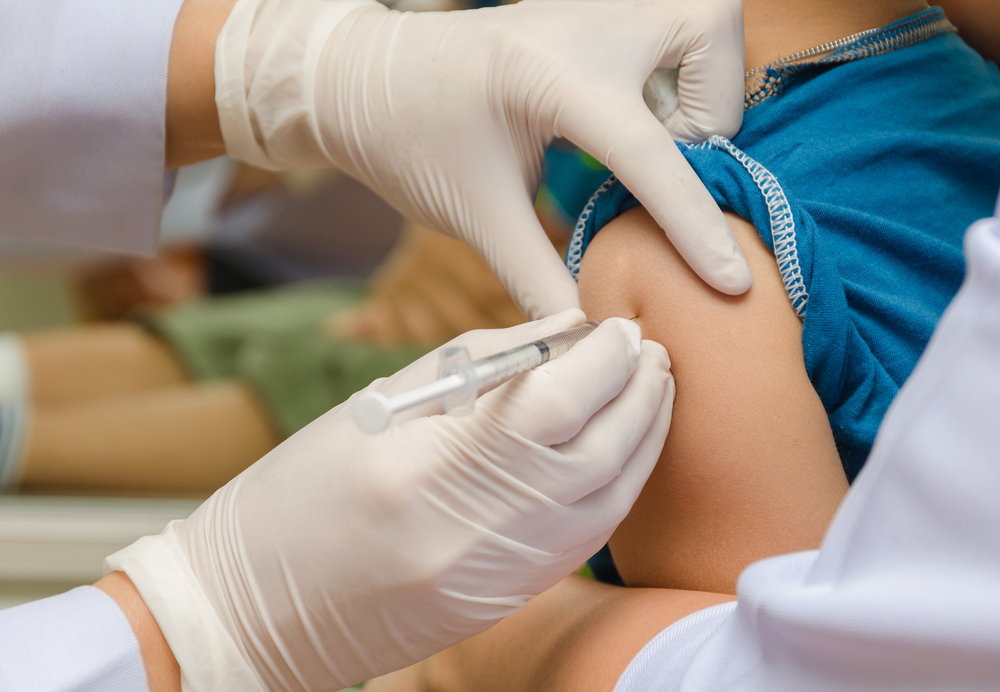
The Vanguard of the Philippines, Inc. (VCPI) and Volunteers Against Crime and Corruption (VACC) on Monday filed administrative cases against 13 incumbent officials of the Department of Health (DOH) in connection with controversial anti-dengue vaccine Dengvaxia. (Shutterstock)
MANILA — The Vanguard of the Philippines, Inc. (VCPI) and Volunteers Against Crime and Corruption (VACC) on Monday filed administrative cases against 13 incumbent officials of the Department of Health (DOH) in connection with controversial anti-dengue vaccine Dengvaxia.
VPCI president Eligio Mallari and VACC representative Atty. Glenn Chong filed the administrative complaint before the Office of the President (OP).
The respondents include DOH Undersecretaries Carol Tanio, Gerardo Bayugo, Lilibeth David and Mario Villaverde; Assistant Secretaries Lyndon Lee Suy and Nestor Santiago; Director Laureano Cruz, OIC Directors Joyce Ducusin and Mar Wyann Bello; Director IV Leonila Gorgolon, Rio Magpantay and Ariel Valencia and Julius Lecciones.
The petitioners asked the OP to “preventively” suspend the respondents “so that they may not use their power or influence to destroy, hide or tamper with the evidence as well as intimidate potential witnesses against them or otherwise obstruct justice.”
The 13 incumbent DOH officials were co-respondents in a complaint filed by VACC before the Commission on Elections last Friday. Others include former President Benigno Aquino III, former DOH Secretary Janette Garin, former Budget Secretary Florencio Abad and four other former DOH officials.
The petitioners accused Aquino and other respondents for violating the Omnibus Election Code since they released the PHP3.5 billion to purchase Dengvacia within 45 days ban before the May 2016 elections.
According to their joint complaint, the petitioners said the respondents are guilty of “grave misconduct and gross negligence for ill-advisedly, thoughtlessly, or imprudently implementing” the dengue immunization program.
The VPCI and VACC claimed that procurement of the Dengvaxia was “irrefutably” fast tracked and may have transgressed pertinent provision of the government procurement law.
A total of 830,000 children and 30,000 more, including members of the Philippine National Police (PNP), received doses of the Dengvaxia vaccine.
According to Dengvaxia manufacturer Sanofi Pasteur, the dengue vaccine should only be administered to children who already contracted dengue aged nine years old and above.
The DOH suspended the vaccination program in December 2017 after Sanofi Pasteur said Dengvaxia poses risks to those without prior infection.
At present, government authorities are investigating the death of 14 children who had been immunized with Dengvaxia.


















Avery23
February 6, 2018 at 5:20 AM
Fire all FDA and DOH officials please!