American News
US regulators approve first digital pill to track patients
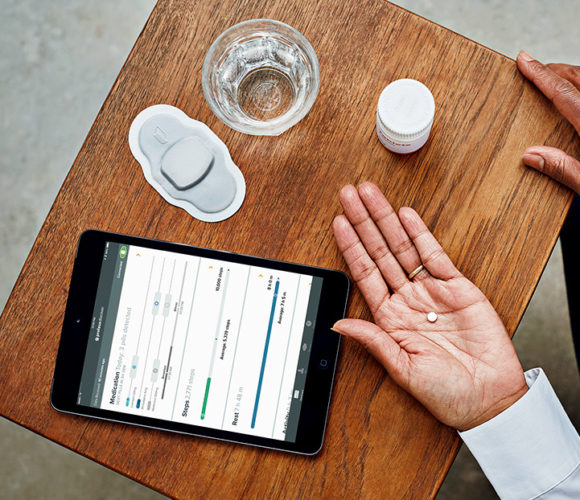
Experts say the technology could be a usefull tool, but it will also change how doctors relate to their patients as they’re able to see whether they are following instructions. (Photo by Proteus Digital Health)
WASHINGTON — U.S. regulators have approved the first drug with a sensor that alerts doctors when the medication has been taken, offering a new way of monitoring patients but also raising privacy concerns.
The digital pill approved Monday combines two existing products: the former blockbuster psychiatric medication Abilify — long used to treat schizophrenia and bipolar disorder — with a sensor tracking system first approved in 2012.
The technology is intended to help prevent dangerous emergencies that can occur when patients skip their medication, such as manic episodes experienced by those suffering from bipolar disorder.
But developers Otsuka Pharmaceutical Co. and Proteus Digital Health are likely to face hurdles. The pill has not yet been shown to actually improve patients’ medication compliance, a feature insurers are likely to insist on before paying for the pill. Additionally, patients must be willing to allow their doctors and caregivers to access the digital information.
These privacy issues are likely to crop up more often as drugmakers and medical device companies combine their products with technologies developed by Silicon Valley.
Experts say the technology could be a useful tool, but it will also change how doctors relate to their patients as they’re able to see whether they are following instructions.
“It’s truth serum time,” said Arthur Caplan, a medical ethicist at NYU’s Langone Medical Center. “Is the doctor going to start yelling at me? Am I going to get a big accusatory speech? How will that interaction be handled?”
The technology carries risks for patient privacy too if there are breaches of medical data or unauthorized use as a surveillance tool, said James Giordano, a professor of neurology at Georgetown University Medical Center.
“Could this type of device be used for real-time surveillance? The answer is of course it could,” said Giordano.
The new pill, Abilify MyCite, is embedded with a digital sensor that is activated by stomach fluids, sending a signal to a patch worn by the patient and notifying a digital smartphone app that the medication has been taken.
The FDA stressed however that there are limitations to monitoring patients.
“Abilify MyCite should not be used to track drug ingestion in ‘real-time’ or during an emergency,” the statement said, “because detection may be delayed or may not occur.”
Patients can track their dosage on their smartphone and allow their doctors, family or caregivers to access the information through a website.
In a statement issued last May at the time the FDA accepted submission of the product for review, the companies said “with the patient’s consent, this information could be shared with their health care professional team and selected family and friends, with the goal of allowing physicians to be more informed in making treatment decisions that are specific to the patient’s needs.”
While it’s the first time the FDA has approved such a pill, various specialty pharmacies and hospitals in the U.S. have previously “packaged” various drugs and sensors. But the federal endorsement increases the likelihood that insurers will eventually pay for the technology.
Drugmakers frequently reformulate their drugs to extend their patent life and to justify raising prices. For instance, Otsuka already sells a long-acting injectable version of Abilify intended to last for one month. The patent on the original Abilify pill expired in 2015.
The Japanese drugmaker has not said how it will price the digital pill. Proteus Digital Health, based in Redwood City California, makes the sensor.